Category:Decision 1082/2013/EU: Serious cross border health threat
This article introduces the serious cross-border health threat legislation, Decision 1082/2013/EU.
This Decision entered into force on 6 November 2013, and is the result of a major progress in health security issues over the last 15 years, in particular:
- The Decision 2119/98/EC on the setting up a network for the epidemiological surveillance and control of communicable diseases,
- The creation in December 2001, following the anthrax scares in the United States, of the Health Security Committee which mandate was to address CBRN events linked to terrorism activities,
- The creation in 2005 of the European Centre for Disease prevention and control, better known as ECDC and based in Stockholm, and which role is to manage the surveillance of communicable diseases, to provide scientific advice and risk assessments on diseases outbreaks, The ECDC hosts and operate, for the Commission, the Early Warning and Response System,
- The entry in force in 2007 of the International Health Regulation developed in 2005 by the World Health Organisation, and which brings into the picture the All Hazard approach, and the development by member States of core capacities,
The legal basis for addressing serious cross-border health threats has been reinforced with the Lisbon Treaty in Article 168. The Treaty stipulates that the EU must complement and support national policies and encourage cooperation between Member States, without superseding their competence in that field. Following number of calls for action from the EU Council and the European Parliament, in December 2011, the Commission came forward with a proposal for a Decision on serious cross border threat to health. The main purpose of this initiative was to extend the protection provided to European citizens to all serious cross border threats to health caused not only by the communicable diseases but by other biological, chemical and environmental threats.
The Decision entered into force on 6 November 2013. It repeals Decision 2119 of 1998, but all implementing acts that were adopted under this Decision remain in force.
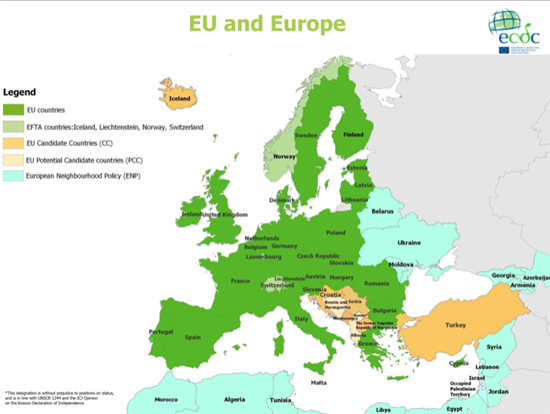
The Decision requires the adoption of further implementing acts and other instruments necessary to implement it properly, like guidelines, technical guidance, standard operational procedures, adoption of IT tools. The Decision includes 33 recitals. They state concisely the reasons for the main provisions of the Decision. They provide the justifications for the measures taken and to assess whether an act is or is not well founded. Recitals reflect upcoming articles or underline important points not reflected specifically in the articles and provide historical background & background to the Decision. The European Court of Justice has ruled that the recitals to a Union act are not legally binding; in particular, they cannot be relied on as a ground for derogating from the provisions of the act. The Court often refers to recitals in order to interpret the enacting terms of an act. Some clarification on key recitals can be found here. Since the objectives of this Decision, cannot be sufficiently achieved by the Member States alone due to the cross-border dimension of serious threats to health and can, therefore, be better achieved at Union level, the Union may adopt measures, in accordance with the principle of subsidiarity as set out in Article 5 of the Treaty on European Union. In accordance with the principle of proportionality, as set out in that Article, this Decision does not go beyond what is necessary in order to achieve those objectives. The recitals also refer to the international dimension induced by the scope of the Decision and informs that the Commission has already the right to conclude international agreements and cooperate with third countries, As such, no specific articles on this issue is needed in the core of the Decision itself, Though the European Union has opened the internal borders between countries to allow free movement of people and goods, borders still demarcate the influence of national authorities in terms of health measures. The cross border health legislation deals aims an facilitating efficient and effective response to health threats between countries. The map below shows EU/EEA Member States (that fall within the influence of this legislation), (potential) candidate countries and neighboring countries.
Contents
Rationale:
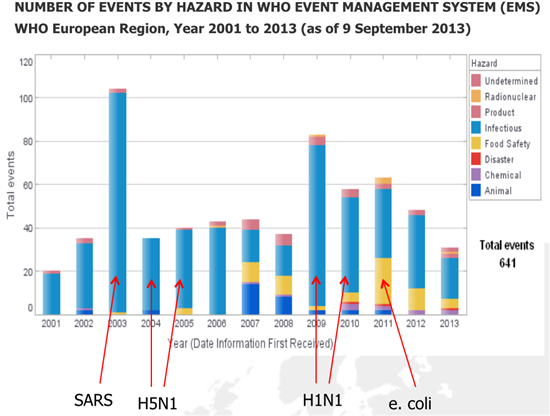
Bridge gaps: address all health threats in a comprehensive and consistent manner reflect evolutions (IHR, ECDC,…) and lessons learnt (H5N1, H1N1, eColi, ) since 1998 New elements:
Strengthened risk preparedness and response planning (e.g. periodic reporting on capacities, all-hazards approach and focus on intersectoral collaboration) Greater access to “medical countermeasures” (e.g. joint procurement of vaccines) Enhanced risk monitoring and assessment (e.g. EWRS for all threats) Improved risk management (e.g. legal basis for HSC) Clearer risk and crisis communication
- Health threats can be:
- threats of biological origin, consisting of:
- communicable diseases;
- antimicrobial resistance and healthcare-associated infections related to communicable diseases ;
- biotoxins or other harmful biological agents not related to communicable diseases;
- threats of chemical origin;
- threats of environmental origin;
- threats of unknown origin;
- events which may constitute public health emergencies of international concern under IHR
- threats of biological origin, consisting of:
- Subsidiarity principle: Europe-wide cooperation when more efficient than individual EU governments acting alone
=> Cross-border nature gives the EU legitimacy to act
- applies to biological, chemical or environmental threats
- existing rules on preparing for and managing health emergencies will be strengthened
- the Health Security Committee will be given a stronger mandate to react in a crisis
Main objectives
- To ensure adequate level of preparedness planning for pandemics and other types of serious cross border health threats across the EU
- To include provisions for joint procurement of medical countermeasures
- To provide for risk assessment and risk management for serious cross border health threats from chemical, biological and environmental origin
- To coordinate EU-wide response and avoid duplication with other instruments at EU and international level (eg. IHR)
‘serious cross-border threat to health’ means a life-threatening or otherwise serious hazard to health of biological, chemical, environmental or unknown origin which spreads or entails a significant risk of spreading across the national borders of Member States, and which may necessitate coordination at Union level in order to ensure a high level of human health protection.
Examples of Public health threats with cross-border relevance:
- Major community-wide outbreak of gastrointestinal disease
- Outbreak of an unknown illness
- Previously unrecognised pathogen in the blood supply
- Chemical, biological, or radiological contamination of a water supply
- Lost source or an accidental release of radiation affecting > 1MS
- An emergent or re-emergent infection abroad that could be imported
- Serious imported infection affecting a number of countries
- International concern over the safety of a vaccine
- Emergence of a new sexually transmitted infection (STI) or the re-emergence of a previously recognised STI
- Influenza pandemic
- Suspected deliberate or accidental release of a serious biological agent
- Major international epizootic with implications for human health
Reference documents
- Decision 1082/2013/EC on Cross Border Health Threats
- ECDC Founding Regulation - 851/2004/EC
FEM PAGE CONTRIBUTORS 2007
- Contributor
- Arnold Bosman
Pages in category "Decision 1082/2013/EU: Serious cross border health threat"
This category contains only the following page.